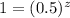Chemistry, 13.08.2019 00:30 dpranavesh446
For the reaction a + b + c → products, the following initial-rate data were obtained. [a]0 (mol/l) [b]0 (mol/l) [c]0 (mol/l) initial rate (mol/(l ∙ s)) 0.40 0.40 0.20 0.0160 0.20 0.40 0.40 0.0080 0.60 0.10 0.20 0.0015 0.20 0.10 0.20 0.0005 0.20 0.20 0.40 0.0020 what are the reaction orders with respect to a, b, and c, respectively? a. 0, 2, 1 b. 0, 1, 1 c. 1, 2, 0 d. 1, 1, 1 e. 1, 2, 1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
You know the right answer?
For the reaction a + b + c → products, the following initial-rate data were obtained. [a]0 (mol/l) [...
Questions

Mathematics, 20.04.2020 00:36

Health, 20.04.2020 00:36


Mathematics, 20.04.2020 00:37

Mathematics, 20.04.2020 00:37

Mathematics, 20.04.2020 00:37


Mathematics, 20.04.2020 00:37

English, 20.04.2020 00:37



Physics, 20.04.2020 00:37

Biology, 20.04.2020 00:37

Mathematics, 20.04.2020 00:37





English, 20.04.2020 00:37

Mathematics, 20.04.2020 00:37

![Rate = k[A]^{x}[B]^{y}[C]^{z}](/tpl/images/0174/5973/51b37.png)
![\frac{Rate3}{Rate4}= [\frac{[A(3)]}{[A(4)]}]^{x}](/tpl/images/0174/5973/34c33.png)
![\frac{0.0015}{0.0005}= [\frac{[0.6]}{[0.2]}]^{x}](/tpl/images/0174/5973/ff890.png)
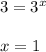
![\frac{Rate2}{Rate5}= [\frac{[B(2)]}{[B(5)]}]^{y}](/tpl/images/0174/5973/f0ddb.png)
![\frac{0.0080}{0.0020}= [\frac{[0.4]}{[0.2]}]^{y}](/tpl/images/0174/5973/14efe.png)
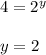
![\frac{Rate1}{Rate2}= [\frac{[A(1)]}{[A(2)]}]^{x}[\frac{[B(1)]}{[B(2)]}]^{y}[\frac{[C(1)]}{[C(2)]}]^{z}](/tpl/images/0174/5973/66487.png)
![\frac{0.0160}{0.0080}= [\frac{[0.4]}{[0.2]}]^{1}[\frac{[0.4]}{[0.4]}]^{2}[\frac{[0.2]}{[0.4]}]^{z}](/tpl/images/0174/5973/ed578.png)