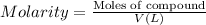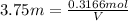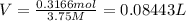Chemistry, 12.08.2019 22:20 thomasgnathan
Hydrogen peroxide can be prepared by the reaction of barium peroxide with sulfuric acid according to the reaction bao2(s)+h2so4(aq)⟶baso4(s)+h2o2(aq) how many milliliters of 3.75 m h2so4(aq) are needed to react completely with 53.5 g bao2(s)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Hydrogen peroxide can be prepared by the reaction of barium peroxide with sulfuric acid according to...
Questions



Mathematics, 23.10.2019 08:50



English, 23.10.2019 08:50

History, 23.10.2019 08:50


German, 23.10.2019 08:50



Mathematics, 23.10.2019 08:50

English, 23.10.2019 08:50




History, 23.10.2019 08:50



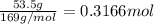
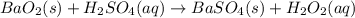
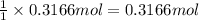 of sulfuric acid.
of sulfuric acid.