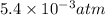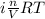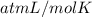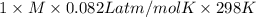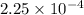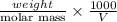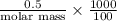Chemistry, 12.08.2019 22:20 cravingnafi202
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and then placed across a semipermeable membrane from a volume of pure water. when the system reaches equilibrium, the solution compartment is elevated 5.6 cm above the solvent compartment. assuming that the density of the solution is 1.0 g / ml, calculate the molecular mass of the unknown.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Asolution of 0.5 g of an unknown nonvolatile, nonelectrolyte solute is added to 100 ml of water and...
Questions



Physics, 06.07.2019 02:00

Mathematics, 06.07.2019 02:00


Social Studies, 06.07.2019 02:00




Physics, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00

Health, 06.07.2019 02:00

History, 06.07.2019 02:00


Mathematics, 06.07.2019 02:00




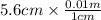 = 0.056, density = 1.0 g/ml, g = 9.8 m/s
= 0.056, density = 1.0 g/ml, g = 9.8 m/s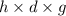
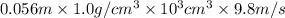
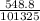 atm
atm