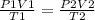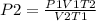Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1


Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20. ∘c . if the container is compressed to 2.60 l and the t...
Questions

English, 14.09.2020 15:01

Chemistry, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Social Studies, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Social Studies, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Biology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01