
Chemistry, 12.08.2019 21:20 edfrank6278
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.500 m solution of aspirin has a ph of 1.86. you are interested in learning about the % dissociation in a buffered solution of aspirin, so you make a new 1.00 l solution containing 0.500 moles of aspirin and 0.25 moles of the sodium salt of aspirin. what will the % dissociation be in this new buffered solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1



Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
In your lab you are studying aspirin, and its acid/base properties. you find that a 1.00 l of a 0.50...
Questions

Mathematics, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00



History, 30.10.2020 01:00



Mathematics, 30.10.2020 01:00

Business, 30.10.2020 01:00

English, 30.10.2020 01:00





Geography, 30.10.2020 01:00

Mathematics, 30.10.2020 01:00


Mathematics, 30.10.2020 01:00


![-log[H^{+}]](/tpl/images/0174/4805/1d5a1.png)
![[H^{+}] = 10^{-pH}](/tpl/images/0174/4805/241df.png)
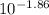

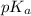 value will be calculated as follows.
value will be calculated as follows. =
= ![\frac{[H^{+}]^{2}}{[Aspirin]}](/tpl/images/0174/4805/0efaa.png)
![\frac{[13.8 \times 10^{-3}]^{2}}{0.50}](/tpl/images/0174/4805/d6d3a.png)

![pK_{a} = -log [K_{a}]](/tpl/images/0174/4805/95c79.png)
![-log [3.8 \times 10^{-4}]](/tpl/images/0174/4805/46d55.png)
![pK_{a} + log \frac{[CH_{3}COO^{-}]}{[Aspirin]}](/tpl/images/0174/4805/cc4ea.png)
![log \frac{[0.25]}{[0.5]}](/tpl/images/0174/4805/52faa.png)
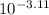
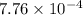 M
M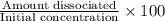

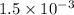 × 100
× 100

