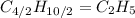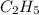Chemistry, 12.08.2019 19:30 lizzyhearts
Determine the empirical formulas for the following compounds:
(a) acetic acid, c24o2
(b) citric acid, c6h8o7
(c) hydrazine, n2h4
(d) nicotine, c10h14n2
(e) butane, c4h10

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Determine the empirical formulas for the following compounds:
(a) acetic acid, c24o2
(b...
(a) acetic acid, c24o2
(b...
Questions



Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

Chemistry, 04.02.2021 21:10

Social Studies, 04.02.2021 21:10


History, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Biology, 04.02.2021 21:10

Biology, 04.02.2021 21:10

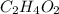 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.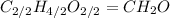
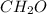
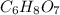 . To write the empirical formula, we divide each subscript by 1.
. To write the empirical formula, we divide each subscript by 1.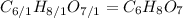
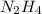 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.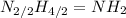
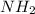
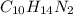 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.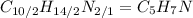
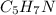
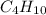 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.