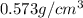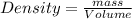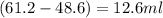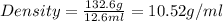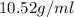Chemistry, 12.08.2019 17:20 jhanley4637
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water. when the jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 ml.
(a) determine the density of this piece of jewelry.
(b) assuming that the jewelry is made from only one suvstance, what substance is it likely to be? explain

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 15:30
Express your answer using two significant figures. 1.7 km^2
Answers: 2
You know the right answer?
Alarge piece of jewelry has a mass of 132.6 g. a graduated cylinder initially contains 48.6 ml water...
Questions





Chemistry, 05.09.2020 17:01

Geography, 05.09.2020 17:01



Mathematics, 05.09.2020 17:01








English, 05.09.2020 17:01