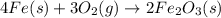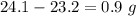Chemistry, 12.08.2019 16:20 novarosell
When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown iron (iii) oxide which we call rust. (a) if a shiny ironnail with an initial mass of 23.2 g is weighed after being coated in a layer of rust, would you expect the mass to have increased, decreased, or remained the same? explain. (b) if the mass of the iron nall increases to 24.1 g what mass of oxygen combined with the iron?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 12:30
Growing crops in places where major pests don't live using beneficial insects to eat harmful insects using a rat trap instead of a rodenticide developing drought-resistant tomato plants using beneficial insects or natural oils to repel pests planting a different crop every year to fake out the pests keeping food covered to deter ants and rodents developing bean plants with a resistance to fungus picking caterpillars off tomato plants cultivation practice biological control cultural control genetic resistance natural chemicals
Answers: 3

Chemistry, 23.06.2019 13:00
How does the kinetic energy of a substance's particle in the solid phase compare to their kinetic enegy in the liquid phase?
Answers: 1

You know the right answer?
When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown iron (i...
Questions

Mathematics, 18.12.2020 20:00

History, 18.12.2020 20:00

Physics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00


Mathematics, 18.12.2020 20:00





Mathematics, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00



French, 18.12.2020 20:00


History, 18.12.2020 20:00

Mathematics, 18.12.2020 20:00

English, 18.12.2020 20:00