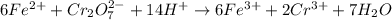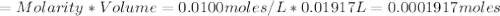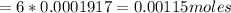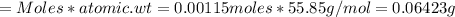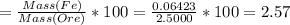Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid solution. a 2.5000-g sample of iron ore is dissolved and the iron converted into iron(ii). exactly 19.17 ml of 0.0100 m na2cr2o7 is required in the titration. what percentage of the ore sample was iron?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Iron(ii) can be oxidized to iron(iii) by dichromate ion, which is reduced to chromium(iii) in acid s...
Questions

Mathematics, 05.03.2021 01:40

Mathematics, 05.03.2021 01:40


Mathematics, 05.03.2021 01:40

Arts, 05.03.2021 01:40

Mathematics, 05.03.2021 01:40



English, 05.03.2021 01:40



Mathematics, 05.03.2021 01:40




Biology, 05.03.2021 01:40

Chemistry, 05.03.2021 01:40

English, 05.03.2021 01:40