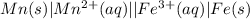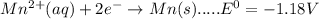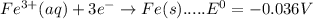Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

You know the right answer?
Consider the following half-reactions and their standard reduction potentials then give the standard...
Questions



Social Studies, 26.10.2019 23:43






Mathematics, 26.10.2019 23:43


Chemistry, 26.10.2019 23:43




Biology, 26.10.2019 23:43



History, 26.10.2019 23:43

Social Studies, 26.10.2019 23:43