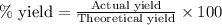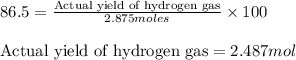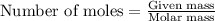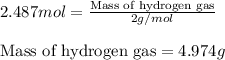Chemistry, 10.08.2019 03:20 jazprincezz7606
If the reaction of 5.75 moles of sodium with excess hydrofluoric acid produced an 86.5% yield of hydrogen gas, what was the actual yield of hydrogen gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
If the reaction of 5.75 moles of sodium with excess hydrofluoric acid produced an 86.5% yield of hyd...
Questions

Mathematics, 21.06.2021 02:40

Mathematics, 21.06.2021 02:40

Mathematics, 21.06.2021 02:40




Mathematics, 21.06.2021 02:40

Mathematics, 21.06.2021 02:40


Mathematics, 21.06.2021 02:40


Mathematics, 21.06.2021 02:40






Mathematics, 21.06.2021 02:40

Mathematics, 21.06.2021 02:40

Mathematics, 21.06.2021 02:40

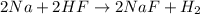
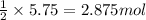 of hydrogen gas.
of hydrogen gas.