
Chemistry, 10.08.2019 00:10 makennskyee1198
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75 m for hydrogen and 0.25 m for ammonia gas, what is the kc? show all work. does this mean that there are more reactants or products at equilibrium? explain how you determined that.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
If the reaction n2 (g) + 3 h2 (g) --> 2 nh3 (g) has the concentrations 1.1 m for nitrogen, 0.75...
Questions

English, 31.01.2020 04:04

History, 31.01.2020 04:04


History, 31.01.2020 04:04






Mathematics, 31.01.2020 04:04

Mathematics, 31.01.2020 04:04

Mathematics, 31.01.2020 04:04



English, 31.01.2020 04:04

English, 31.01.2020 04:04


Biology, 31.01.2020 04:04


English, 31.01.2020 04:04

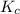 is 0.136 and is reactant favored.
is 0.136 and is reactant favored.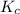
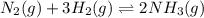
![K_{c}=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0173/9631/f3b94.png)
![[NH_3]=0.25M](/tpl/images/0173/9631/306b2.png)
![[H_2]=0.75M](/tpl/images/0173/9631/5c336.png)
![[N_2]=1.1M](/tpl/images/0173/9631/4c5ce.png)
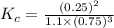
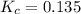
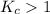 ; the reaction is product favored.When
; the reaction is product favored.When 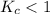 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 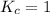 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

