

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

You know the right answer?
For the reaction 2 a ⇌ b, the equilibrium concentrations are as follows: [a] = 0.056 mand [b] = 0.1...
Questions

Mathematics, 19.08.2021 21:50








History, 19.08.2021 21:50

Mathematics, 19.08.2021 21:50



Mathematics, 19.08.2021 21:50

Mathematics, 19.08.2021 21:50






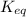 .
.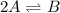
![K_{eq} = \frac{[B]}{[A]^{2}}](/tpl/images/0173/8965/36e57.png)
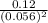
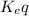 ) for the reaction is 38.
) for the reaction is 38.

