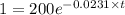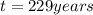Chemistry, 09.08.2019 22:30 miabarrett8117
Cesium-137 has a half-life of about 30 years. given this half-life, we can represent its decay with the exponential decay function a=a0e(ln(0.5)30)t if we begin with 200 mg of cesium-137, how long will it take for the cesium to decay to the point where there is only 1 milligram remaining? round to the closest year.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

You know the right answer?
Cesium-137 has a half-life of about 30 years. given this half-life, we can represent its decay with...
Questions


Mathematics, 17.12.2020 23:30

Computers and Technology, 17.12.2020 23:30

Social Studies, 17.12.2020 23:30

English, 17.12.2020 23:30




Mathematics, 17.12.2020 23:30

Chemistry, 17.12.2020 23:30


Mathematics, 17.12.2020 23:30

Mathematics, 17.12.2020 23:30

Biology, 17.12.2020 23:30

Chemistry, 17.12.2020 23:30

Arts, 17.12.2020 23:30


Computers and Technology, 17.12.2020 23:30

History, 17.12.2020 23:30

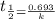
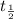 = half life = 30 years
= half life = 30 years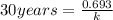
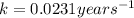
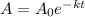
![[A_o]](/tpl/images/0173/9051/dc622.png) = Concentration of reactant initially = 200 mg
= Concentration of reactant initially = 200 mg![[A]](/tpl/images/0173/9051/6aa06.png) = Concentration of reactant at time t = 1 mg
= Concentration of reactant at time t = 1 mg