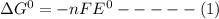Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o. the reaction has a δg°' value of –81 kj/mol. the negative value of δg°' results from the very e°' value of the redox couple no3–/no2–, and it reflects the strong tendency of nitrate to electrons. choose one: a. positive / donateb. negative/ acceptc. negative / donated. positive / accept

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
Consider the following half reaction in which nitrate is reduced to nitrite: no3– 2h 2e– → no2– h2o...
Questions


Biology, 02.07.2019 17:20



Biology, 02.07.2019 17:20


Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20

Biology, 02.07.2019 17:20


Biology, 02.07.2019 17:20



Biology, 02.07.2019 17:20



Mathematics, 02.07.2019 17:30