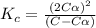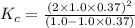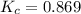Chemistry, 09.08.2019 19:20 pwolfiimp4
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium described by the equation n2o4(g) ↔ 2no2(g) if at equilibrium the n2o4 is 37% dissociated, what is the value of the equilibrium constant, kc, for the reaction under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1


Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Exactly 1.0 mol n2o4 is placed in an empty 1.0-l container and is allowed to reach equilibrium descr...
Questions

Mathematics, 25.01.2021 20:50



Social Studies, 25.01.2021 20:50






Mathematics, 25.01.2021 20:50

Mathematics, 25.01.2021 20:50

Biology, 25.01.2021 20:50


Biology, 25.01.2021 20:50




Mathematics, 25.01.2021 20:50


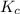 for the reaction is, 0.869
for the reaction is, 0.869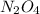 .
.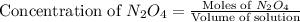
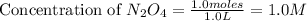
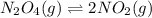
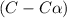
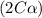
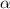 = 37 % = 0.37
= 37 % = 0.37![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0173/7935/271f5.png)