
Chemistry, 29.01.2020 20:53 winchester729
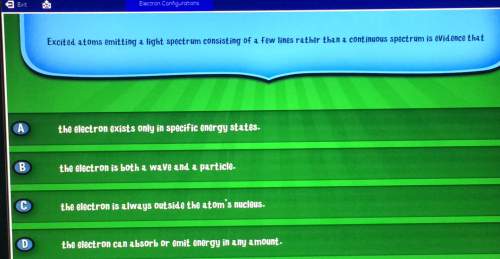
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather than a continuous spectrum is evidence thatcad the electron exists only in specific energy statesb the electron is both a wave and a particleco the electron is always outside the atom's nucleus. d the electron can absorb or emit energy in any amount

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Electron configurationsexitexcited atoms emitting a light spectrum consisting of a few lines rather...
Questions




Mathematics, 13.12.2020 02:30

Social Studies, 13.12.2020 02:30

Social Studies, 13.12.2020 02:30


Computers and Technology, 13.12.2020 02:30


Chemistry, 13.12.2020 02:30




Mathematics, 13.12.2020 02:30

English, 13.12.2020 02:30

Arts, 13.12.2020 02:30



Mathematics, 13.12.2020 02:30

History, 13.12.2020 02:30



