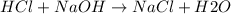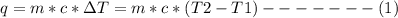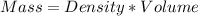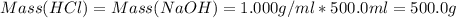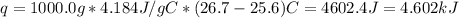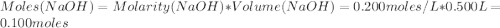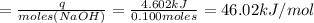Chemistry, 09.08.2019 04:10 Hamadsaqer9
Avolume of 500.0 ml of 0.220 m hcl(aq) was added to a high quality constant-pressure calorimeter containing 500.0 ml of 0.200 m naoh(aq). both solutions have a density of 1.000 g ml-1 and a specific heat of 4.184 j g‑1 oc-1. the temperature of the entire system rose from 25.60 °c to 26.70 °c. calculate the heat of reaction, in kj, per mole of naoh(aq).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Avolume of 500.0 ml of 0.220 m hcl(aq) was added to a high quality constant-pressure calorimeter con...
Questions


Biology, 17.09.2019 20:00

Mathematics, 17.09.2019 20:00

Mathematics, 17.09.2019 20:00


History, 17.09.2019 20:00




Health, 17.09.2019 20:00


Social Studies, 17.09.2019 20:00

English, 17.09.2019 20:00

Computers and Technology, 17.09.2019 20:00


Physics, 17.09.2019 20:00

Physics, 17.09.2019 20:00


English, 17.09.2019 20:00

English, 17.09.2019 20:00