
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilibrium mixture of this reaction results in the formation of more h2s? which change to an equilibrium mixture of this reaction results in the formation of more ? a decrease in the volume of the reaction vessel (at constant temperature) an increase in the amount of nh4hs in the reaction vessel an increase in temperature all of the above

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
The decomposition of nh4hs is endothermic: nh4hs(s)⇌nh3(g)+h2s(g) part a which change to an equilib...
Questions

Mathematics, 06.05.2020 05:59

Mathematics, 06.05.2020 05:59

Physics, 06.05.2020 05:59




Mathematics, 06.05.2020 05:59


Mathematics, 06.05.2020 05:59





Mathematics, 06.05.2020 05:59


Advanced Placement (AP), 06.05.2020 05:59




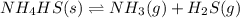
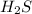 will increase.
will increase.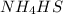 then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.
then equilibrium will shift in the direction of decrease in concentration that is, in the forward direction.

