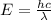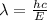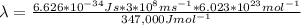The carbon-carbon (c―c) bond has an average bond energy of 347 kj/mol, which is the energy required to break one mole of c―c bonds. what is the wavelength of the photon that can break this bond? 487 nm 457 nm 354 nm 345 nm 377 nm
(1) 457 nm
(2) 487 nm
(3) 345 nm
(4) 354 nm
(5) 377 nm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
The carbon-carbon (c―c) bond has an average bond energy of 347 kj/mol, which is the energy required...
Questions

Mathematics, 16.04.2021 22:50

Mathematics, 16.04.2021 22:50



Mathematics, 16.04.2021 22:50



Mathematics, 16.04.2021 22:50

Health, 16.04.2021 22:50

History, 16.04.2021 22:50

Mathematics, 16.04.2021 22:50

History, 16.04.2021 22:50



Mathematics, 16.04.2021 22:50

Medicine, 16.04.2021 22:50

Mathematics, 16.04.2021 22:50

Biology, 16.04.2021 22:50

Mathematics, 16.04.2021 22:50