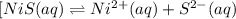Chemistry, 08.08.2019 18:10 ayannabrewer4408
Which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of ni2+ is increased? view available hint(s) which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of is increased? ni(oh)2(s)⇌ni2+(aq)+2oh−(aq) [ni(h2o)6]2+(aq)+3en(aq)⇌[ni(en)3]2 +(aq)+6nh3(aq) ni2+(aq)+6nh3(aq)⇌[ni(nh3)6]2+ nis(s)⇌ni2+(aq)+s2−(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

You know the right answer?
Which equilibrium reaction will experience a shift towards the products in equilibrium position when...
Questions





World Languages, 14.07.2021 20:50

Health, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50


Mathematics, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50



French, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50




Chemistry, 14.07.2021 20:50

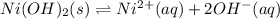
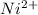 is increased in this reaction then reaction will shift in a direction that will be opposing the change. This means that the reaction will shift in backward direction.
is increased in this reaction then reaction will shift in a direction that will be opposing the change. This means that the reaction will shift in backward direction.![[Ni(H_{2}O)_{6}]^{2+}(aq) + 3en(aq) \rightleftharpoons [Ni(en)_{3}]^{2+}(aq) + 6NH_{3}(aq)](/tpl/images/0173/2468/316d9.png)
![[Ni^{2+}(aq) + 6NH_{3}(aq) \rightleftharpoons [Ni(NH_{3})_{6}]^{2+}(aq)](/tpl/images/0173/2468/fdf11.png)