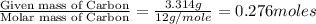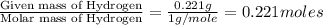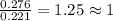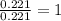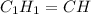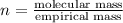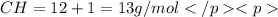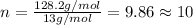Chemistry, 08.08.2019 06:20 alwaysneedhelp84
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams of co2 and 1.990 grams of h2o were produced.
in a separate experiment, the molar mass of the compound was found to be 128.2 g/mol. determine the empirical formula and the molecular formula of the hydrocarbon.
enter the elements in the order presented in the question.
empirical formula =
molecular formula =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
when 3.539 grams of a hydrocarbon, cxhy, were burned in a combustion analysis apparatus, 12.15 grams...
Questions


English, 24.11.2020 01:40



Mathematics, 24.11.2020 01:50


Mathematics, 24.11.2020 01:50


Biology, 24.11.2020 01:50

History, 24.11.2020 01:50

Mathematics, 24.11.2020 01:50


Mathematics, 24.11.2020 01:50

Mathematics, 24.11.2020 01:50



History, 24.11.2020 01:50

Mathematics, 24.11.2020 01:50


Mathematics, 24.11.2020 01:50

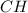 and
and 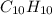 respectively.
respectively.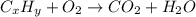
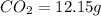
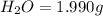
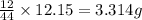 of carbon will be contained.
of carbon will be contained.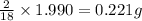 of hydrogen will be contained.
of hydrogen will be contained.