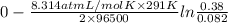Aconcentration cell is built based on the following half reactions by using two pieces of zinc as electrodes, two zn2+ solutions, 0.129 m and 0.427 m, and all other materials needed for a galvanic cell. what will the potential of this cell be when the cathode concentration of zn2+ has changed by 0.047 m at 291 k?
zn2+ + 2 e- ? zn eo = -0.761 v

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Aconcentration cell is built based on the following half reactions by using two pieces of zinc as el...
Questions

Engineering, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20



Mathematics, 10.02.2021 23:20

Biology, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20

Mathematics, 10.02.2021 23:20





English, 10.02.2021 23:20


Health, 10.02.2021 23:20

Social Studies, 10.02.2021 23:20



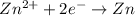 ,
, 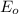 = -0.761 V
= -0.761 V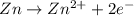 ,
, 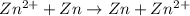
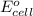 for the given reaction is zero.
for the given reaction is zero.![E^{o}_{cell} - \frac{RT}{nF} ln \frac{[Zn^{2+}]_{products}}{[Zn^{2+}]_{reactants}}](/tpl/images/0173/1699/4d9c9.png)