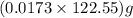8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g) + 2kcio, (s) the oxygen was collected by the displacement of water at 22'c at a total pressure of 754 torr. the volume of the gas collected was 0.65l and the vapor pressure of water at 22°c is 21 torr. calculate a) the partial pressure of o2 in the gas collected and b) the mass of kcio3 in the sample that was decomposed

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1


Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
8. a sample of potassium chlorate (kcio,) was heated in a test tube and decomposed 2kc? (s) 302 (g)...
Questions


Mathematics, 22.04.2021 06:50


Mathematics, 22.04.2021 06:50

Mathematics, 22.04.2021 06:50


Social Studies, 22.04.2021 06:50

Mathematics, 22.04.2021 06:50

Mathematics, 22.04.2021 06:50



Biology, 22.04.2021 06:50






Mathematics, 22.04.2021 06:50


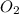 in the gas was 733 torr and mass of
in the gas was 733 torr and mass of 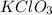 in the sample was 2.12 g.
in the sample was 2.12 g.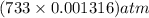 = 0.9646 atm
= 0.9646 atm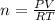
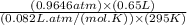
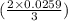 moles or 0.0173 moles of
moles or 0.0173 moles of