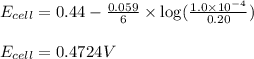Chemistry, 08.08.2019 05:30 katier9407
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
(a) write the three balanced equations (each half-cell and the overall):
(b) what is the standard cell potential (e°cell) for this mn/cr cell?
(c) what will be the cell potential when [mn2+] = 0.20 m and [cr3+] = 1.0 × 10-4 m ?
(d) what will be the cell potential when [mn2+] = 1.0 × 10-4 m and [cr3+] = 0.20 m ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Nernst equation – for the following reaction:
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
mn (s) + cr3+ (aq) ? mn2+ (aq) + cr (s)
...
Questions

Mathematics, 11.05.2021 22:20


Chemistry, 11.05.2021 22:20

Social Studies, 11.05.2021 22:20

Business, 11.05.2021 22:20


Physics, 11.05.2021 22:20


Mathematics, 11.05.2021 22:20

Geography, 11.05.2021 22:20






English, 11.05.2021 22:20

Mathematics, 11.05.2021 22:20


Mathematics, 11.05.2021 22:20

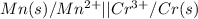
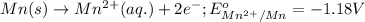 ( × 3)
( × 3)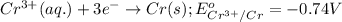 ( × 2)
( × 2)
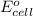 of the reaction, we use the equation:
of the reaction, we use the equation: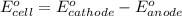
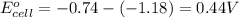
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]}{[Cr^{3+}]}](/tpl/images/0173/1570/b6936.png)
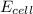 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Cr^{3+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/252be.png)
![[Mn^{2+}]=0.20M](/tpl/images/0173/1570/1716f.png)
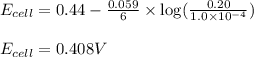
![[Cr^{3+}]=0.20M](/tpl/images/0173/1570/cae64.png)
![[Mn^{2+}]=1.0\times 10^{-4}M](/tpl/images/0173/1570/83dbd.png)