
Chemistry, 08.08.2019 04:20 vipergod07
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentrations as a result of increasing air pollution. the two processes for the natural removal of co2 from the atmosphere are photosynthesis and dissolution into the oceans. complete the following chemical equation for the dissolution of co2 in water. remember to include the physical states of the products. co. lag) +h what happens to the ph of the ocean as more co2 dissolves? oincreases o decreases o no change

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Carbon dioxide (co2) is an abundant greenhouse gas that is present in high atmospheric concentration...
Questions






Mathematics, 27.06.2020 02:01


Mathematics, 27.06.2020 02:01









Mathematics, 27.06.2020 02:01



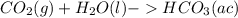 ; The pH decrease.
; The pH decrease.

