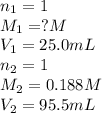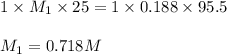Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

You know the right answer?
By titration, it is found that 95.5 ml95.5 ml of 0.188 m naoh(aq)0.188 m naoh(aq) is needed to neutr...
Questions

Computers and Technology, 29.07.2019 16:40

Mathematics, 29.07.2019 16:40


Business, 29.07.2019 16:40






Biology, 29.07.2019 16:40



Biology, 29.07.2019 16:40




Health, 29.07.2019 16:40



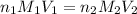
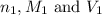 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl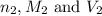 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.