
Chemistry, 08.08.2019 00:20 aroland1990x
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. δ h°f [caco3(s)] = –1206.9 kj/mol; δ h°f [cao(s)] = –635.1 kj/mol; δ h°f [co2(g)] = –393.5 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
Calculate the δ h°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide...
Questions



Law, 20.04.2020 21:01

Mathematics, 20.04.2020 21:01



Mathematics, 20.04.2020 21:01


English, 20.04.2020 21:02


Mathematics, 20.04.2020 21:02


Mathematics, 20.04.2020 21:02





Mathematics, 20.04.2020 21:02



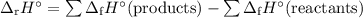
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ}& = & [(-635.1 + (-393.5)] - (-1206.9)\\& = & -1028.6 +1206.9\\& = & \mathbf{178.3}\\\end{array}\\\text{The enthalpy of decomposition is } \boxed{\textbf{178.3 kJ/mol}}](/tpl/images/0172/9753/c40b5.png) l}}
l}}



