
Chemistry, 07.08.2019 06:10 makeda2010
A125 g sample of iron (iii) bromide, febr3, is weighed out on a balance. (a) how many moles of the compound are present? (b) how many iron (iii) ions are present in the sample? (c) how many ions of bromide are present in the sample? an excellent response will clearly show each step in your reasoning, indicating the units (dimensions) of the answers and consideration of significant figures. for answers greater than 1000 or less than 0.1 scientific notation should be used.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

You know the right answer?
A125 g sample of iron (iii) bromide, febr3, is weighed out on a balance. (a) how many moles of the c...
Questions




Mathematics, 26.02.2020 01:31





History, 26.02.2020 01:31




Mathematics, 26.02.2020 01:31

Mathematics, 26.02.2020 01:31

Mathematics, 26.02.2020 01:31

Mathematics, 26.02.2020 01:31

Mathematics, 26.02.2020 01:31


History, 26.02.2020 01:31

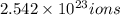 of iron(III)
of iron(III)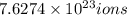 of bromide.
of bromide.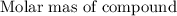
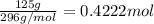
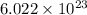 molecules
molecules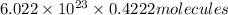
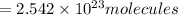 of iron (III) bromide
of iron (III) bromide 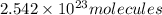 will contain:
will contain: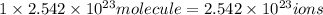 of iron(III)
of iron(III)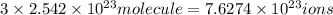 of bromide.
of bromide.

