
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the following concentrations are present: [so2] = 0.010 m; [so3] = 10.m; [o2] = 0.010 m. is the mixture at equilibrium? if not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium? yes, the mixture is at equilibrium. no, left to right. no, right to left. there is not enough information to be able to predict the direction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
At 700 k, the reaction2so2(g) + o2(g) 2so3(g)has the equilibrium constant kc = 4.3 x 106, and the fo...
Questions

Mathematics, 28.01.2020 05:31

History, 28.01.2020 05:31


History, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31

Chemistry, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31


Biology, 28.01.2020 05:31

Social Studies, 28.01.2020 05:31


Biology, 28.01.2020 05:31





Mathematics, 28.01.2020 05:31

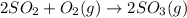
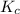 = 4.3 \times 10^{6}[/tex].
= 4.3 \times 10^{6}[/tex].![[SO_2]](/tpl/images/0172/4735/0303a.png) = 0.010 M;
= 0.010 M; ![[SO_3]](/tpl/images/0172/4735/8a06f.png) = 10.M;
= 10.M; ![[O_2]](/tpl/images/0172/4735/b0db0.png) = 0.010 M.
= 0.010 M.![\frac{[SO_{3}]^{2}}{[SO_{2}]^{2}[O_{2}]}](/tpl/images/0172/4735/2750c.png)
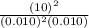
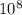
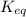 , then reaction moves in the backward direction.
, then reaction moves in the backward direction.

