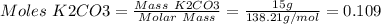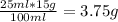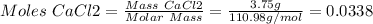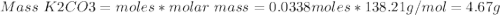Chemistry, 06.08.2019 19:30 santosbeti90
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and calcium chloride. 2. using this balanced equation, determine the limiting reactant if 15 grams of calcium chloride was reacted with 15 grams of potassium carbonate. 3. using your answer for question 2, determine the mass of potassium carbonate needed to fully precipitate all the calcium from a 25 ml sample of 15% calcium chloride.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
1. write a balanced equation for the precipitation of calcium carbonate from potassium carbonate and...
Questions

Mathematics, 04.03.2021 09:30


Geography, 04.03.2021 09:30

Mathematics, 04.03.2021 09:30



Mathematics, 04.03.2021 09:30



Mathematics, 04.03.2021 09:30


Chemistry, 04.03.2021 09:30



Mathematics, 04.03.2021 09:30

Mathematics, 04.03.2021 09:30

Mathematics, 04.03.2021 09:30

Mathematics, 04.03.2021 09:30


Physics, 04.03.2021 09:30

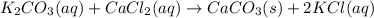
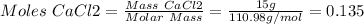
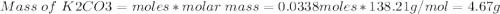 = 15 g
= 15 g