
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient (q) is greater than k, reactant must be converted to products to reach equilibrium.2. for a chemical system at equilibrium, the forward and reverse rates of reaction are equal.3. for a chemical system at equilibrium, the concentrations of products divided by the concentrations of reactants equals one.1 only2 only3 only1 and 21, 2, and 3

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Which of the following statements is/are correct? 1. for a chemical system, if the reaction quotient...
Questions






Mathematics, 24.06.2019 13:50


Mathematics, 24.06.2019 13:50

Mathematics, 24.06.2019 13:50




Computers and Technology, 24.06.2019 13:50







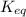 , then it means that the reaction is proceeding in the backward reaction. Whereas if Q <
, then it means that the reaction is proceeding in the backward reaction. Whereas if Q < 

