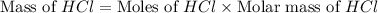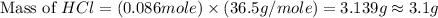Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30.0 g of hcl (fw = 36.5 g/mol) react according to the following chemical equation? mno2 + 4 hcl ® mncl2 + cl2 + 2 h2o3.1 g hcl23.3 g hcl4.02 g mno28.0 g mno212.1 g mno2

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Which reactant, and how many grams of it, is left over after 16.0 g of mno2 (fw = 86.9 g/mol) and 30...
Questions

French, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00


English, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00




Social Studies, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00

Health, 23.01.2022 14:00

Geography, 23.01.2022 14:00





Social Studies, 23.01.2022 14:00

Mathematics, 23.01.2022 14:00

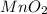 = 16.0 g
= 16.0 g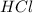 = 30.0 g
= 30.0 g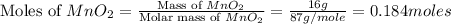
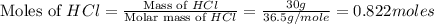
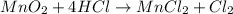
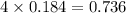 moles of
moles of