
Chemistry, 06.08.2019 04:20 kenzielema12
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha , and dilutes to the mark with distilled water. the technician then titrates this weak acid solution with 0.0969 m koh . she reaches the endpoint after adding 43.81 ml of the koh solution. determine the number of moles of the weak acid in the solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
To a 25.00 ml volumetric flask, a lab technician adds a 0.150 g sample of a weak monoprotic acid, ha...
Questions

Mathematics, 26.02.2020 02:52



Computers and Technology, 26.02.2020 02:52










Computers and Technology, 26.02.2020 02:52

Mathematics, 26.02.2020 02:52

Mathematics, 26.02.2020 02:52

Computers and Technology, 26.02.2020 02:52


Mathematics, 26.02.2020 02:52

Mathematics, 26.02.2020 02:52

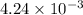 moles.
moles.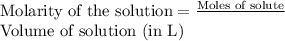
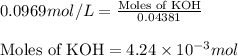
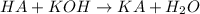
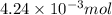 of KOH will react with =
of KOH will react with = 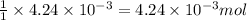 of weak monoprotic acid.
of weak monoprotic acid.

