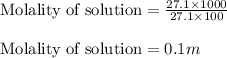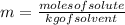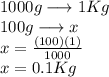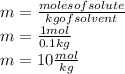Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
If you combine 27.1 g of a solute that has a molar mass of 27.1 g/mol with 100.0 g of a solvent, wha...
Questions




English, 12.11.2020 09:40


Mathematics, 12.11.2020 09:40


Mathematics, 12.11.2020 09:40


Spanish, 12.11.2020 09:40


Computers and Technology, 12.11.2020 09:40

English, 12.11.2020 09:40

Mathematics, 12.11.2020 09:40



Mathematics, 12.11.2020 09:40


Mathematics, 12.11.2020 09:40

Computers and Technology, 12.11.2020 09:40

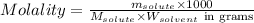
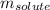 = Given mass of solute = 27.1 g
= Given mass of solute = 27.1 g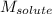 = Molar mass of solute = 27.1 g/mol
= Molar mass of solute = 27.1 g/mol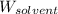 = Mass of solvent = 100 g
= Mass of solvent = 100 g