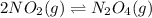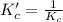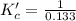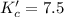The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625 mole of n2o4 was introduced into a 5.00 l vessel and was allowed to decompose until equilibrium was reached. the concentration of n2o4 at equilibrium was 0.0750 m. calculate kc for the reaction.0.0500.07500.100.1257.5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 13:00
Johnny's bakery has 30,900 grams of sugar. a recipe calls for 32 pounds of sugar to be used. how much sugar will be left over? (1 lb=453.59 g).
Answers: 2
You know the right answer?
The brown gas no2 and the colorless gas n2o4 exist in equilibrium,2no2 n2o4.in an experiment, 0.625...
Questions


English, 28.08.2019 10:30

Physics, 28.08.2019 10:30

Chemistry, 28.08.2019 10:30

History, 28.08.2019 10:30

Mathematics, 28.08.2019 10:30

English, 28.08.2019 10:30


History, 28.08.2019 10:30

Mathematics, 28.08.2019 10:30


History, 28.08.2019 10:30


Mathematics, 28.08.2019 10:30



Biology, 28.08.2019 10:30

Mathematics, 28.08.2019 10:30


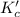 for the reaction is, 7.5
for the reaction is, 7.5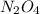 =
= 
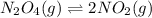
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0172/0236/271f5.png)
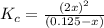
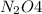 at equilibrium = 0.0750 M
at equilibrium = 0.0750 M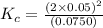
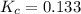
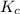 for the given reaction.
for the given reaction.