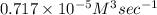Consider the reaction: 3a + b + 2 c -> d + e where rate = k[a]2 [c]2 .a reaction was performed where [b]o = [c]o = 0.600 m and [a]o = 2.20 x 10-5 m. a plot of 1/[a] vs time (min) gave a plot with a straight line relationship and after 7.00 min, [a] = 2.4 x 10-6 m. what was the initial rate of the reaction described above?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1
You know the right answer?
Consider the reaction: 3a + b + 2 c -> d + e where rate = k[a]2 [c]2 .a reaction was performed...
Questions




History, 25.07.2019 18:40



Geography, 25.07.2019 18:40


Mathematics, 25.07.2019 18:40

Biology, 25.07.2019 18:40




Social Studies, 25.07.2019 18:40






Mathematics, 25.07.2019 18:40

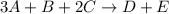
![[A]^{2}[C]^{2}](/tpl/images/0171/8736/1432d.png)
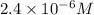 and
and ![[A]_{0} = <img src=](/tpl/images/0171/8736/27003.png)
![\frac{1}{[A]} = \frac{1}{[A]_{0}} + kt](/tpl/images/0171/8736/0bb6a.png)
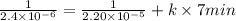
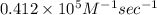
![[A]^{2}_{o} [C]^{2}_{o}](/tpl/images/0171/8736/e40c6.png)