
Chemistry, 27.08.2019 03:00 angelteddy033
What is the arrhenius definition of a base? a substance that increases h3o+ concentration when it is dissolved in water. a substance that increases oh– concentration when it is dissolved in water. a compound that donates protons. a compound that accepts protons

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1


Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2
You know the right answer?
What is the arrhenius definition of a base? a substance that increases h3o+ concentration when it i...
Questions

Mathematics, 11.07.2019 14:30




Chemistry, 11.07.2019 14:30

History, 11.07.2019 14:30



Computers and Technology, 11.07.2019 14:30


History, 11.07.2019 14:30

Geography, 11.07.2019 14:30



Biology, 11.07.2019 14:30

History, 11.07.2019 14:30


Computers and Technology, 11.07.2019 14:30

Physics, 11.07.2019 14:30

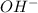 concentration when it is dissolved in water.
concentration when it is dissolved in water.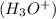 in an aqueous solution. In other words, a substance dissociates in water to form
in an aqueous solution. In other words, a substance dissociates in water to form 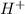 ion.Arrhenius Base : It increases the concentration of hydroxide ion
ion.Arrhenius Base : It increases the concentration of hydroxide ion 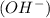 in an aqueous solution. In other words, a substance dissociates in water to form
in an aqueous solution. In other words, a substance dissociates in water to form 

