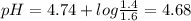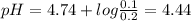Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Be sure to answer all parts. calculate the ph of the following two buffer solutions: (a) 1.4 m ch3c...
Questions


Computers and Technology, 24.07.2019 04:00


History, 24.07.2019 04:00


History, 24.07.2019 04:00


Spanish, 24.07.2019 04:00


Social Studies, 24.07.2019 04:00


English, 24.07.2019 04:00

Biology, 24.07.2019 04:00

English, 24.07.2019 04:00





English, 24.07.2019 04:00

![pH =pKa +log\frac{[salt]}{[acid]}](/tpl/images/0171/7883/d188a.png)