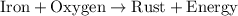Chemistry, 03.08.2019 03:10 koolbeast68
Rust formation is represented as iron + oxygen → rust + energy. which describes this reaction?
rust formation is an exothermic reaction because it absorbs energy.
rust formation is an exothermic reaction because it releases energy.
rust formation is an endothermic reaction because it absorbs energy.
rust formation is an endothermic reaction because it releases energy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Rust formation is represented as iron + oxygen → rust + energy. which describes this reaction?
Questions

Mathematics, 25.12.2019 11:31


Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31


Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31


Computers and Technology, 25.12.2019 12:31


Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

Mathematics, 25.12.2019 12:31

History, 25.12.2019 12:31


English, 25.12.2019 12:31