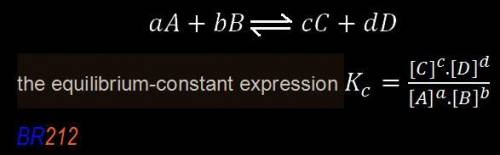Chemistry, 03.08.2019 02:30 sainijasdeep27
Write the equilibrium‑constant expression for the reaction a(s)+3b(l)↽−−⇀2c(aq)+d(aq) in terms of [a], [b], [c], and [d], as needed. note that , which is sometimes symbolized as , denotes that the equilibrium constant is expressed using molar concentrations. for this question, means the same thing as .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1


Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
Write the equilibrium‑constant expression for the reaction a(s)+3b(l)↽−−⇀2c(aq)+d(aq) in terms of [a...
Questions

Mathematics, 10.11.2020 20:10


Mathematics, 10.11.2020 20:10



Physics, 10.11.2020 20:10




Mathematics, 10.11.2020 20:10





Mathematics, 10.11.2020 20:10

Business, 10.11.2020 20:10

Mathematics, 10.11.2020 20:10


English, 10.11.2020 20:10

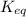
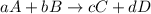
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0163/9721/9c8b0.png)
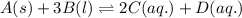
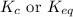 for the given reaction follows:
for the given reaction follows:![K_{c}=\frac{[C]^2[D]^1}{[A]^1[B]^3}](/tpl/images/0163/9721/f02fe.png)
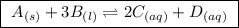 , we can observe that the substances on the right-hand side have a solution phase (aq) that is allowed into the equilibrium constant K.
, we can observe that the substances on the right-hand side have a solution phase (aq) that is allowed into the equilibrium constant K.![\boxed{ \ K = \frac{[C]^2.[D]}{[A].[B]^3} \ }](/tpl/images/0163/9721/e01b1.png) we get the final result
we get the final result ![\boxed{ \ K = [C]^2.[D] \ }](/tpl/images/0163/9721/83dc2.png)
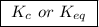 , denotes that the equilibrium constant is expressed using molar concentrations, i.e.,
, denotes that the equilibrium constant is expressed using molar concentrations, i.e., 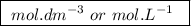 . For this question,
. For this question, 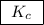 means the same thing as
means the same thing as 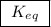 .The rIght-hand side of the equation on top, left-hand side of the equation on the bottom.The square brackets show concentrations in
.The rIght-hand side of the equation on top, left-hand side of the equation on the bottom.The square brackets show concentrations in