
Chemistry, 03.08.2019 02:10 mariaaaaa69
Suppose 0.210kg of octane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0°c. calculate the volume of carbon dioxide gas that is produced. round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1



Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Suppose 0.210kg of octane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0...
Questions

Mathematics, 18.08.2019 16:20

Social Studies, 18.08.2019 16:20

Physics, 18.08.2019 16:20


History, 18.08.2019 16:20

English, 18.08.2019 16:20



English, 18.08.2019 16:20

Mathematics, 18.08.2019 16:20

Biology, 18.08.2019 16:20


Biology, 18.08.2019 16:20


World Languages, 18.08.2019 16:20


Chemistry, 18.08.2019 16:20

History, 18.08.2019 16:20

History, 18.08.2019 16:20


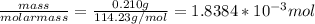
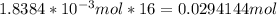
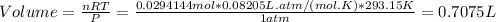
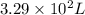
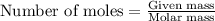
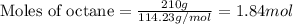
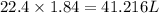 of volume.
of volume.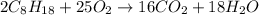
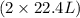 of octane gas produces
of octane gas produces 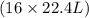 of carbon dioxide gas.
of carbon dioxide gas.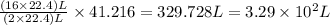 of carbon dioxide gas.
of carbon dioxide gas.

