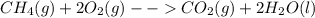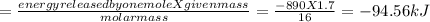Chemistry, 03.08.2019 00:20 jtorres0520
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon dioxide and water: ch4 (g) 2o2 (g) → co2 (g) 2h2o(l) δh = -890.0 kj
calculate the value of q (kj) in this exothermic reaction when 1.70 g of methane is combusted at constant pressure.
(a) -94.6 kj
(b) 0.0306 kj
(c) -0.0106 kj
(d) 32.7 kj
(e) -9.46 × 10^4 kj

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
In the presence of excess oxygen, methane gas burns in a constant-pressure system to yield carbon di...
Questions

Chemistry, 21.03.2021 23:30

English, 21.03.2021 23:30



Mathematics, 21.03.2021 23:30

Mathematics, 21.03.2021 23:30

History, 21.03.2021 23:30


Biology, 21.03.2021 23:30

Mathematics, 21.03.2021 23:30

English, 21.03.2021 23:30


Mathematics, 21.03.2021 23:30







Social Studies, 21.03.2021 23:40