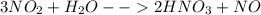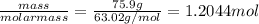Chemistry, 03.08.2019 00:10 chancecharles9oug353
Consider the following balanced reaction. how many grams of water are required to form 75.9 g of hno3? assume that there is excess no2 present. the molar masses are as follows: h2o = 18.02 g/mol, hno3 = 63.02 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Consider the following balanced reaction. how many grams of water are required to form 75.9 g of hno...
Questions



Mathematics, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10

Arts, 15.12.2020 06:10

Spanish, 15.12.2020 06:10

Spanish, 15.12.2020 06:10



Mathematics, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10

History, 15.12.2020 06:10

Health, 15.12.2020 06:10


Mathematics, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10

World Languages, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10

Mathematics, 15.12.2020 06:10