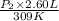Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
Consider 4.60 l of a gas at 365 mmhg and 20 c . if the container is compressed to 2.60 l and the tem...
Questions

Computers and Technology, 22.08.2019 03:20

Social Studies, 22.08.2019 03:20

History, 22.08.2019 03:20

Social Studies, 22.08.2019 03:20




Computers and Technology, 22.08.2019 03:20







Mathematics, 22.08.2019 03:20

Physics, 22.08.2019 03:20



Mathematics, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20

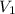 = 4.60 L,
= 4.60 L, 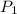 = 365 mm Hg
= 365 mm Hg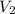 = 2.60 L,
= 2.60 L, 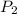 = ?
= ?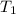 = (20 + 273) K = 293 K,
= (20 + 273) K = 293 K, 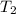 = (36 + 273) K = 309 K
= (36 + 273) K = 309 K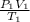 =
= 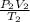
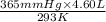 =
=