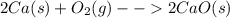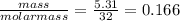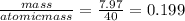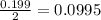Chemistry, 02.08.2019 23:20 chloeozenghar1
Many metals react with oxygen gas to form the metal oxide. for example, calcium reacts as follows: 2ca(s) + o2(g) → 2cao(s) calculate the mass of calcium oxide that can be prepared from 7.97 g of ca and 5.31 g of o2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 16:30
Amodel of an atom is shown below. which element is represented by this model of an atom? boron, carbon, neon, or sodium?
Answers: 1

Chemistry, 24.06.2019 00:00
Ascientist performs an experiment where she measures the mass of a piece of metal, pours acid onto the metal, washes the metal, and then re-measures the mass of the metal. the scientist repeats the experiment several times, each time increasing the amount of acid used. the dependent variable in the experiment is the: initial mass of the metal final mass of the metal amount of acid used washing the metal
Answers: 1
You know the right answer?
Many metals react with oxygen gas to form the metal oxide. for example, calcium reacts as follows:...
Questions



Biology, 07.07.2019 16:30





Biology, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30


Mathematics, 07.07.2019 16:30

Physics, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30


Social Studies, 07.07.2019 16:30




Chemistry, 07.07.2019 16:30