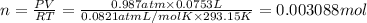Chemistry, 02.08.2019 22:20 shongmadi77
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory experiment. the reaction is 2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g). the hydrogen gas is collected over water. how many moles of hydrogen gas were formed when 75.3 ml is collected at 20.0oc and 768.0 torr pressure? the vapor pressure of water at this temperature is 17.5 torr.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2


Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
The reaction of solid aluminum with hydrochloric acid is used to make hydrogen gas in a laboratory e...
Questions

English, 23.07.2019 14:00


Biology, 23.07.2019 14:00

Social Studies, 23.07.2019 14:00







Spanish, 23.07.2019 14:00

Chemistry, 23.07.2019 14:00

Mathematics, 23.07.2019 14:00

Mathematics, 23.07.2019 14:00

Spanish, 23.07.2019 14:00