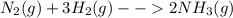Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1
You know the right answer?
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammon...
Questions

Mathematics, 29.10.2020 21:10

Health, 29.10.2020 21:10



Mathematics, 29.10.2020 21:10



History, 29.10.2020 21:10



Mathematics, 29.10.2020 21:10


Mathematics, 29.10.2020 21:10

History, 29.10.2020 21:10



History, 29.10.2020 21:10


Mathematics, 29.10.2020 21:10