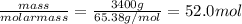Chemistry, 02.08.2019 19:10 abbeygrace13
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is zn2+(aq)+2e−→zn(s) for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.40 kg on the surface to get adequate coverage. part a how many moles of zinc are in 3.40 kg of zinc? express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions

Biology, 20.09.2019 19:40





Biology, 20.09.2019 19:50


English, 20.09.2019 19:50


Spanish, 20.09.2019 19:50

Biology, 20.09.2019 19:50





Biology, 20.09.2019 19:50




History, 20.09.2019 19:50