

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Agaseous hydrogen and carbon containing compound is decomposed and formed to contain 82.66% carbon a...
Questions

English, 27.10.2020 21:10

Business, 27.10.2020 21:10

Mathematics, 27.10.2020 21:10



Mathematics, 27.10.2020 21:10




Health, 27.10.2020 21:10


Mathematics, 27.10.2020 21:10




Mathematics, 27.10.2020 21:10

Geography, 27.10.2020 21:10


English, 27.10.2020 21:10

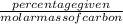
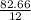
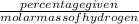
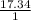
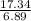 = 2.5
= 2.5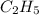 .
.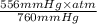
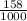 equals 0.158 L.
equals 0.158 L.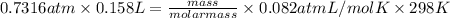
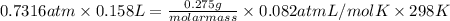
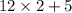 = 29.
= 29.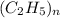 = 58
= 58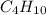 .
.

